Key points
- All of Us received an FDA investigational device exemption to deliver health-related DNA research results to participants.
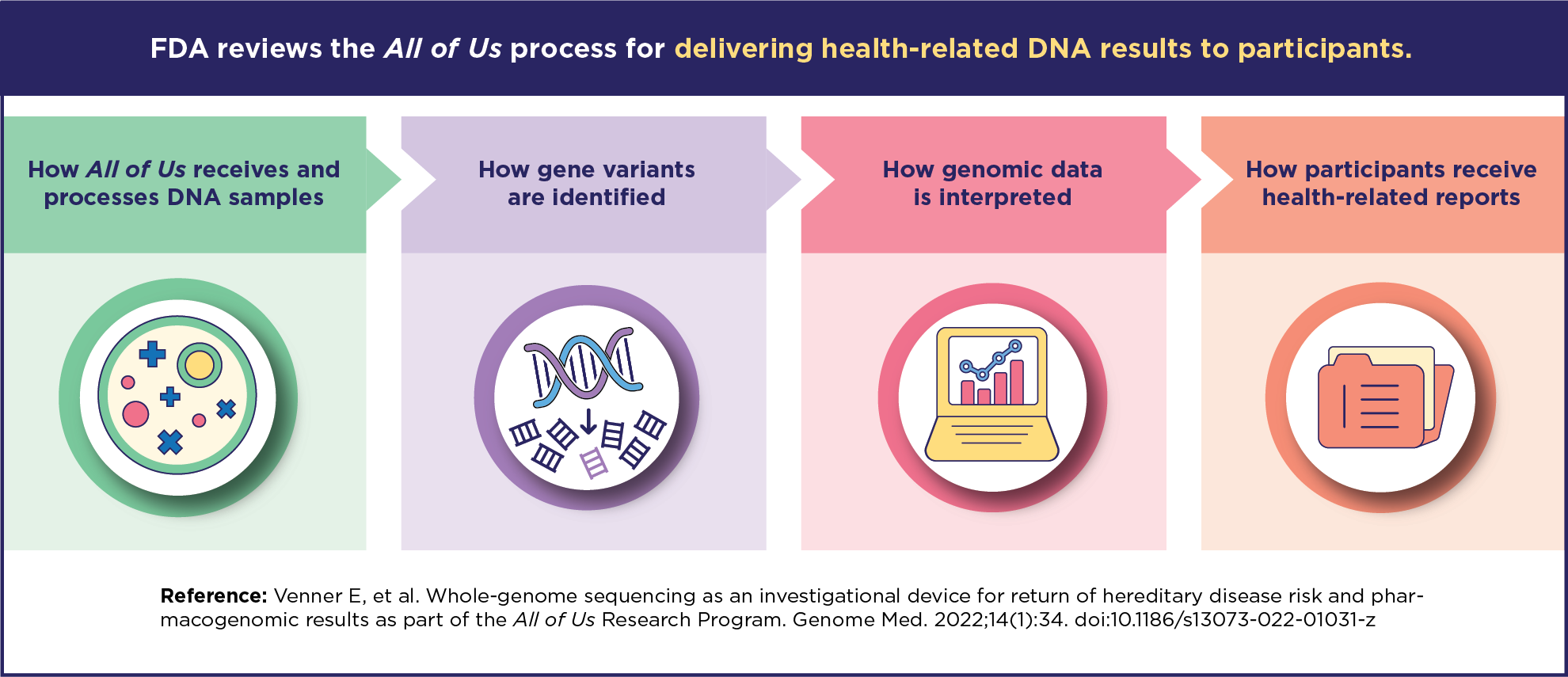
- This process includes four key steps: sequencing whole genomes, identifying gene variants, interpreting data, and creating participant reports.
The All of Us Research Program seeks to collect health data, including DNA information, from one million people across the United States.
In return, the program will deliver health-related DNA results in easy-to-read reports to every participant who wants them. Since the program will give this information directly to participants, All of Us worked with the U.S. Food and Drug Administration (FDA).
In a recent issue of Genome Medicine, researchers at All of Us genome centers describe how they collected data for an investigational device exemption. An investigational device exemption means that we have met the FDA's standards to return health-related research results to our participants.
Why DNA Reports Need FDA Review
The health-related DNA reports from All of Us are based on whole genome sequencing of participants’ DNA. Whole genome sequencing uses technology to look at almost every part of a person’s DNA. To create these reports, All of Us will look for a specific set of genetic variants that are known to increase risk for certain inherited diseases and other variants that can affect how our bodies process certain medicines.
Health-related DNA reports from All of Us are considered research results, not a source of medical advice or care. Because they will be delivered directly to participants, these reports are still required to meet FDA standards. Any findings from these research reports should be confirmed with clinical testing before participants make any changes to their care.
Verifying and Validating the All of Us Process
The research team worked with the All of Us Institutional Review Board and FDA for more than a year and a half to create and test the procedures that will be used to provide personalized health-related DNA results to participants. The team’s efforts included discussions with FDA of every major element of the program process, including:
- Participant consent
- Sample processing
- DNA analysis and interpretation
- Return of results to participants
All of Us used more than 1,200 blood samples to test its process for returning health-related DNA results. This process includes four key steps:
- Sequencing whole genomes
- Identifying gene variants
- Interpreting data
- Designing and generating participant reports
Results showed that All of Us genome centers consistently reproduced their own results. The centers also performed consistently across sites. These findings showed the FDA that the program is able to return consistent results back to participants regardless of which All of Us genome center sequenced their sample.
Evaluation also showed that All of Us methods exceeded 99% for precision and accuracy. Precision means the methods showed the same DNA variant at the same spot every time it was sequenced. Accuracy means the methods read the DNA without error. Precision and accuracy are essential to making sure each participant’s DNA results are reported correctly.
Few research programs have written about going through an FDA review like this one. The All of Us team published this article to share their experience with other researchers who may want to return results to participants in the future.
In addition to sharing results with participants, All of Us makes data from DNA available to researchers for their studies. Learn more about the program’s data platform, the Researcher Workbench.
Interested in All of Us?
- Read more research highlights.
- Learn about participation in the program.
Conduct research with All of Us
- Learn about opportunities for researchers.
- Find funding to support research using All of Us data.
- See more research projects made possible by All of Us data and tools.